Our ProductsLN-008 siMASP2

siRNA Drug Development Program – siMASP2, LN-008
The MASP2 gene plays an important role in the activation of the complement system of the innate immune defense, via the lectin pathway. MASP2 is expressed exclusively in hepatocytes and, as such, is an excellent target for inhibition using a GalNac conjugated siRNA. The target is fully validated in human with neutralizing monoclonal antibodies. Inhibition of the lectin pathway was shown to be a potential therapeutic approach for a remarkable variety of pathological conditions, both orphan and non-orphan, including diseases associated with ischemia-reperfusion injury of various organs and systems, inflammatory vascular pathology, including various types of vasculitis and atherosclerosis ([xii], [xiii],[xiv],[xv]).
The Company is developing a siRNA drug targeting MASP2 with convenient subcutaneous self-administration with infrequent (once in 5-6 months) dosing regimen.
LN-008 is a siRNA inhibiting the MASP2 gene, appropriately chemically modified for efficient delivery to the liver. Proof of concept in human of inhibiting MASP2 for treatment of human diseases was already achieved by antibodies for the MASP2 protein in a number of diseases, including chronic diseases of the kidney, substantially de-risking LN-008 development.
The siRNA drug development program in general and, in particular, the LN-008 program exploits inherent advantages of siNRAs, in general and especially of those that target the liver, over antibodies for the same target.
- Lepton has the know-how and the technology to target siRNA to the liver
- Single (or infrequent) subcutaneous injection
- Duration of effect up to 6 months
- High knockdown efficiency
- Favorable safety profile
The Current siRNA – based Drug Pipeline
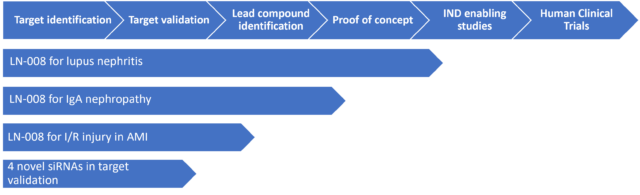
Initial Program: Rare Kidney Diseases
Based on a vast amount of literature [iii], [iv] and clinical trials [v] data with anti MASP2 monoclonal antibody the Company has selected to pursue initially Lupus Nephritis (LN) an inflammation of the kidney caused by systemic lupus erythematosus (SLE or lupus) [vii]that is an autoimmune disease and IgA nephropathy, an autoimmune, slowly progressive rare kidney disease [vi] . Both diseases lead to kidney failure. There’s no cure for either disease and sure way of predicting progression; the goal of treatment is to avoid the need for kidney dialysis or kidney transplantation for as long as possible [viii],[ix].
IgA nephropathy
IgAN is an orphan, progressive, chronic disease, for which there is a high unmet medical need and no approved treatments. It is the most common form of glomerulonephritis worldwide with marked epidemiological differences between regions. The disease reduces life expectancy and leads to kidney failure in 20–40% of patients within 20 years of diagnosis ([xvi]). Systematic reviews of published studies spanning multiple countries suggest an overall population incidence of 1.29 -2.5 per 100,000 with IgAN diagnosis rates ranging from 6.3% to 29.7% among adult and pediatric patients undergoing renal biopsy, ([xvii],[xviii]). IgA nephropathy is observed in up to 40% of all biopsies performed for glomerular disease in Asia, compared with 20% in Europe and 10% in North America. ([xix])
Lupus nephritis
LN is an inflammatory kidney disease caused by the autoimmune disease systemic lupus erythematosus (SLE) and is clinically evident in 50-60% (up to 75%) of patients with SLE, ([xx]). It may be histologically evident in most SLE patients, even without clinical manifestations of renal disease. Treatment of LN usually involves immunosuppressive therapy; however, these treatments are not uniformly effective and may cause comorbidities such as infections, osteoporosis, and cardiovascular and reproductive effects ([xxi],[xxii]). Within 10 years of an initial SLE diagnosis, 10–30% of patients with LN develop ESRD ([xxiii],[xxiv]). The prevalence of SLE and the risk of LN varies considerably between different regions of the world and different races ([xxv]) . In the USA the prevalence of SLE was found to be 81 – 144 per 100,000 based on billing records of 81 million US patients. The prevalence of LN was estimated to be, on average, 20 per 100,000 ([xxvi])
Remarkable Additional Indications
These include non-orphan and orphan indications associated with ischemia-reperfusion injury of various organs and systems, inflammatory vascular pathology including various types of vasculitis and atherosclerosis, rheumatoid heart disease, arthritis and others. A selected future indication is ischemia reperfusion injury (I/R) following acute myocardial infarction (AMI), a leading cause of morbidity and mortality worldwide. It has been shown that MASP-2 dependent activation of the lectin pathway is critically involved in mediating myocardial I/R injury, contributes to continued myocardial injury during reperfusion following AMI [x],[xi] making the inhibition of the lectin pathway, a promising future therapeutic approach in AMI.
References
[i] https://covid.cdc.gov/covid-data-tracker/#demographics
[ii]Java et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5(15):e140711
[iii] Alghadban, Samy et al. “Absence of the Lectin Activation Pathway of Complement Ameliorates Proteinuria-Induced Renal Injury.” Frontiers in immunology vol. 10 2238. 23 Sep. 2019, doi:10.3389/fimmu.2019.02238
[iv] Mariana Gaya da Costa, Felix Poppelaars, Stefan P Berger, Mohamed R Daha, Marc A Seelen, The lectin pathway in renal disease: old concept and new insights, Nephrology Dialysis Transplantation, Volume 33, Issue 12, December 2018, Pages 2073–2079, https://doi.org/10.1093/ndt/gfy073
[v] Clinical trials.gov for Narsoplimab
[vi] Jennifer C. Rodrigues, Mark Haas and Heather N. Reich, CJASN April 2017, 12 (4) 677-686; DOI: https://doi.org/10.2215/CJN.07420716
[vii] Saxena, Ramesh et al. “Lupus nephritis: current update.” Arthritis research & therapy vol. 13,5 (2011): 240. doi:10.1186/ar3378
[viii] https://www.mayoclinic.org/diseases-conditions/lupus-nephritis/diagnosis-treatment/drc-20446438
[ix] https://www.mayoclinic.org/diseases-conditions/iga-nephropathy/diagnosis-treatment/drc-20352274
[x] Clark JE, Dudler T, Marber MS, et alCardioprotection by an anti-MASP-2 antibody in a murine model of myocardial infarctionOpen Heart 2018;5: e000652. doi: 10.1136/openhrt-2017-000652
[xi] Panagiotou Anneza, Trendelenburg Marten, Osthoff Michael, The Lectin Pathway of Complement in Myocardial Ischemia/Reperfusion Injury—Review of Its Significance and the Potential Impact of Therapeutic Interference by C1 Esterase Inhibitor, Frontiers in Immunology. Vol 9, 2018, page 1151, https://www.frontiersin.org/article/10.3389/fimmu.2018.01151, DOI=10.3389/fimmu.2018.01151, ISSN=1664-3224
[xii] Széplaki et al. Role of complement in the pathomechanism of atherosclerotic vascular diseases (2009). Mol Immunol. 46:2784
[xiii] Peerschke et al. Complement Activation on Platelets: Implications for Vascular Inflammation and Thrombosis. (2010). Mol Immunol. 47:2170
[xiv] Beltrame et al. The lectin pathway of complement and rheumatic heart disease. (2015). Front Pediatr. 2:148
[xv] Fumagalli et al. Lectin Complement Pathway and Its Bloody Interactions in Brain Ischemia. (2016). Stroke, 47:3067
[xvi] Rizk DV, Maillard N, Julian BA, Knoppova B, Green TJ, Novak J, Wyatt RJ. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol. 2019 Mar 19;10:504. doi: 10.3389/fimmu.2019.00504. PMID: 30941137; PMCID: PMC6433978
[xvii] Kwon CS, Daniele P, Forsythe A, Ngai C. A Systematic Literature Review of the Epidemiology, Health-Related Quality of Life Impact, and Economic Burden of Immunoglobulin A Nephropathy. JHEOR. 2021;8(2):36-45. doi:10.36469/001c.26129
[xviii] McGrogan A, Franssen CF, de Vries CS, Nephrol Dial Transplant. 2011 Feb; 26(2):414-30. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature; Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402–2414
[xix] Medscape , January 13, 2022, https://www.medscape.com/answers/239927-81332/what-is-the-global-prevalence-of-immunoglobulin-a-iga-nephropathy
[xx] Laura Barcia-Sixto,David Isenberg, Trends in Urology & Men’s Health, Volume 11, Issue 1 anuary/February 2020Pages 26-29
[xxi] Anders, HJ., Saxena, R., Zhao, Mh. et al. Lupus nephritis. Nat Rev Dis Primers 6, 7 (2020). https://doi.org/10.1038/s41572-019-0141-9
[xxii] Irastorza et al, Nefrologia Vol. 32. Issue. S1. January 2012 pages 1-35, DOI: 10.3265/Nefrologia.pre2011. Dec.11298 Full text access
[xxiii] Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci 2013;346:319–23
[xxiv] Luis F. Quintana, David Jayne, Sustained remission in lupus nephritis: still a hard road ahead, Nephrology Dialysis Transplantation, Volume 31, Issue 12, 9 December 2016, Pages 2011–2018, https://doi.org/10.1093/ndt/gfv381
[xxv] Update on Lupus Nephritis, Salem Almaani, Alexa Meara, Brad H. Rovin, CJASN May 2017,12 (5) 825-835; DOI: 10.2215/CJN.05780616
[xxvi] Prevalence Of Systemic Lupus Erythematosus and Lupus Nephritis In The United States: Analysis Of Commercial and Public Insurance Billing Data Gandhi et al.. 2013 ACR/ARHP Annual Meeting, ABSTRACT NUMBER: 1071, https://acrabstracts.org/abstract/prevalence-of-systemic-lupus-erythematosus-and-lupus-nephritis-in-the-united-states-analysis-of-commercial-and-public-insurance-billing-data


