Our ProductsLN-Cast-01

Cell Therapy Program, LN-Cast 01
LN-Cast, Lepton’s proprietary, miRNA-based technology platform (the “Castling Technology”), is a breakthrough technology for manipulation/engineering of miRNA expression by application of Gene Editing Technologies (GETs),in particular CRISPER-CAS9, to create ex vivo, products that modify gene expression for enhanced efficacy and longevity (reduced exhaustion) of cell-based therapies, such as Adoptive Cell Transfer – mediated therapies. The platform is protected by Lepton’s PCT patent application.
The method is in essence, counter-manipulating/ modifying the microRNAs (miRNAs)’ expression patterns imposed by the disease, by editing their genetic loci, ex vivo, in cells, such as T-cells, to improve their functionality. This is performed by simultaneously up-regulating a desired (“beneficial” or “good”) miRNA and shutting down/ down-regulating an undesired (“harmful” or “bad”) miRNA, in a single editing event. The miRNA pair is carefully selected such that the “harmful” member of the pair is transcriptionally induced in pathological state and orchestrates activation of gene network(s) that mediate(s) the unwanted phenotype(s); and the “beneficial” member is under expressed or unchanged in the pathology but, if upregulated ectopically, can activate gene network(s) that counteract(s) the unwanted pathological phenotype(s).
Initially the Castling Technology will be used to achieve better T-cell functionality in cancer treatment. The initial indications contemplated to be developed are blood cancers. Diffuse Large B-cell Lymphoma (DLBCL) is an example of such indication. Several LN-Cast permutations were identified and are examined.
The CAR T-cell therapy success rate is today about 30% to 40% for lasting remission[i]. Broad use of CAR T-cell therapies is limited by biological and logistical drawbacks, including potential for life-threatening toxicities, challenges related to manufacturing a patient-specific product, high costs and inadequate reimbursement, and incomplete or unstained disease response caused, among others, by the exhaustion of the immune cells.[ii].
With its carefully selected miRNA pairs, Lepton’s LN-Cast potentially could overcome the inadequacy of current CAR-T cell therapies for cancer, in particular exhaustion of CAR-T cells, via a single editing event, thus maintaining CAR –T cells persistence and ability to secret pro-inflammatory cytokines.
The simultaneous and reciprocal impact on genetic networks regulated by two different miRNAs is expected to have more significant therapeutic effect compared to single manipulations, improve safety and the cost effectiveness of the treatment.
Please link here for a brief overview of the technology. The Company has accomplished the in vitro POC of the novel technology.
The Current Cell Therapy Pipeline
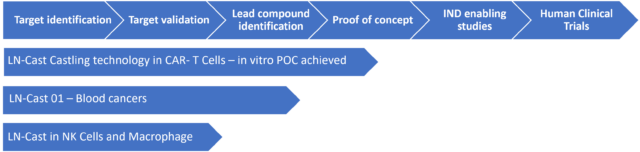
Remarkable Potential Products Based on LN-Cast
- LN- Cast- 01 for Blood Cancers. One of the first indications contemplated is Diffuse Large B-cell Lymphoma (DLBCL). DLBCL is a fast-growing, aggressive form of Non-Hodgkin Lymphoma (NHL) that is fatal within months if left untreated. It is the most common type of NHL diagnosed in the Western hemisphere, representing 30-40% of all NHL cases diagnosed every year in the United States. The incidence and rate of deaths per 100,000 of DLBCL is 5.6 respectively 1.8 per p year[iii]. With treatment, the mean overall survival at 5 years is currently 63.8% in the USA ([ix]). High-dose immunotherapy followed by autologous stem cell transplantation is the standard care for relapsed/refractory (RR) patients with DLBCL. However, >60% of patients are ineligible for a transplant, presenting a therapeutic challenge. [iv] standard CAR T therapies have been approved, however, this therapy is also associated with unexpected toxicities that can be life-threatening [v] [vi].
- Additional LN-Cast permutations of miRNA pairs (also suitable for stable allogenic or off the shelf CAR T cells) for the treatment of:
- Solid tumors
- Potentially additional blood cancers, such as refractory Hodgkin’s a cancer of the lymphatic system [vii] with an incidence close to 9000 cases and almost 1000 deaths registered yearly in the USA [viii] as well as lymphoma, acute myeloid leukemia, multiple myeloma
- The technology is amenable for therapeutic modification of a variety of cell therapies used for treatment of cancer, autoimmune and inflammatory diseases:
- miRNA castling in T-regulatory (Treg) or CAR-Treg cells for the treatment of systemic lupus erythematosus (SLE) and/or allergic asthma
- Chimeric antigen receptor (CAR) T-cells (both autologous or allogeneic)
- CAR-Natural Killer (NK) cells
- CAR-macrophages
- Tumor infiltrating lymphocytes (TILs)
- Mesenchymal stem cells (MSC)
- Additional LN-Cast permutations of miRNA pairs (also suitable for stable allogenic or off the shelf CAR T cells) for the treatment of:
- Solid tumors
- Potentially additional blood cancers, such as refractory Hodgkin’s lymphoma, acute myeloid leukemia, multiple myeloma
- The technology is amenable for therapeutic modification of a variety of cell therapies used for treatment of cancer, autoimmune and inflammatory diseases:
- miRNA castling in T-regulatory (Treg) or CAR-Treg cells for the treatment of systemic lupus erythematosus (SLE) and/or allergic asthma
- Chimeric antigen receptor (CAR) T-cells (both autologous or allogeneic)
- CAR-Natural Killer (NK) cells
- CAR-macrophages
- Tumor infiltrating lymphocytes (TILs)
- Mesenchymal stem cells (MSC)
References
[i] https://www.uchicagomedicine.org/forefront/cancer-articles/a-walking-miracle-car-t-cell-therapy
[ii] Rafiq, S., Hackett, C.S. & Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 17, 147–167 (2020). https://doi.org/10.1038/s41571-019-0297-y
[iii] Cancer Stat Facts: NHL — Diffuse Large B-Cell Lymphoma (DLBCL), National Cancer Institute https://seer.cancer.gov/statfacts/html/dlbcl.html
[iv] Al-Mansour, Mubarak et al. “Efficacy and safety of second-generation CAR T-cell therapy in diffuse large B-cell lymphoma: A meta-analysis.” Molecular and clinical oncology vol. 13,4 (2020): 33. doi:10.3892/mco.2020.2103
[v] Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729.
[vii] https://www.cancer.org/cancer/hodgkin-lymphoma/about/key-statistics.html
[viii] https://archive.cancerworld.net/e-grandround/immunotherapy-in-relapsed-refractory-hodgkin-lymphoma/#:~:text=The%20treatment%20of%20Hodgkin%20lymphoma,will%20eventually%20die%20of%20it.
[ix] https://seer.cancer.gov/statfacts/html/dlbcl.html


